"Beats Flex-fragment design special edition" is now on sale on Apple's official website
11/03/2022

Yuichi Hidaka (Hidaka): We are a bio -venture in Nagoya, so today we will talk about "bio -venture", and then the company outline, followed by the company's development pipeline.I would like to give a research project, and finally explain the growth strategy.
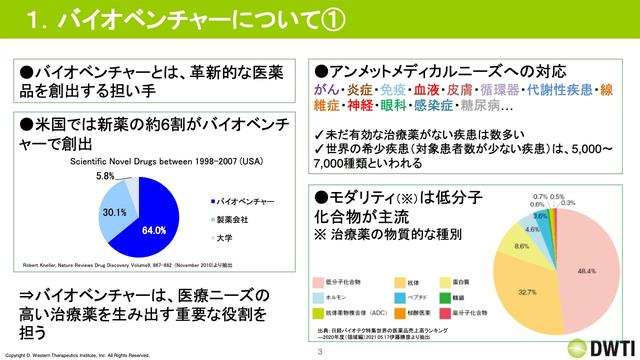
Hidaka: I think it is often said that bio ventures are difficult to understand, so I will explain the bio venture first.At present, the roles of the drugs are becoming quite clear in the pharmaceutical industry, and the bio -venture plays a role in creating and developing drug seeds, and is being sold by pharmaceutical companies.In the United States, some data has created about 60 % of new drugs.
Against this background is that the diseases that are still low in treatment satisfaction and few patients who are still low in treatment have recently been focused.In addition, as a whole flow, technological innovation has progressed, and quite a lot of technologies have emerged, and bio -ventures with sharp techniques make drug seeds because they cannot overtake multiple technologies by pharmaceutical companies alone.It is.
Hidaka: Bioventure is making drug seeds in the pharmaceutical industry, but this industry is growing very much because of the growth industry.In recent years, the price of drug innovation has increased the price of drugs, but the number of patients has increased due to aging, and the number of patients has increased, indicating a very high growth rate.
On the other hand, it is a growing industry, but it is very risky compared to other industries.In short, the probability of successful medicine is low.In other words, you have to spend a lot of money, as a result, you will invest a lot and continue to be in the red, which does not make any medicine.
The graph at the bottom right of the slide is data on how many medicines can be made over 100 billion yen, the vertical axis is the number of drugs and the horizontal axis is chronological.Around 1960, about 100 billion yen was made, about 10 medicines were produced, but around 2000 years, 100 billion yen could not make one, that is, below 1.
Therefore, it is the current state of new drug development that it does not succeed even if a very large amount of money moves, and it is characteristic of the industry that it grows but is high in risk.Here is the significance of the bio venture.
Hidaka: I will give you an overview of the company.We have the headquarters in Nagoya City, Aichi Prefecture, and have a research institute in Mie University.It was established in February 1999 and has been around 22 years.There are 15 employees, and it is often said that it is quite small, but in the same industry, there are many companies with 20 to 30 employees, so I think it is normal.In addition, as a consolidated subsidiary, we own one company called Nippon Leather Leather Drug Counterfeit with Rohto Pharmaceutical.
Hidaka: Next, I will tell you the characteristics of our company.Our founding philosophy is to "the groundbreaking new drugs from Japan to the world", and we are aiming for that.Our basic technology is a technology that makes something called "kinase inhibitor" and is the longest in its technology.Currently, the target disease is currently used as an ophthalmologist.
I will explain it because it is often asked, "What is a kinase inhibitor?"There are various targets of drugs, and some have a huge impact on adjusting protein activities, but the enzyme that is responsible for this on / off switching is Kinase.There is a kinase inhibitor that acts to suppress activity because this kinase is excessive.We are developing a business that uses this as a medicine artificially and artificially.
The reason why we are making kinase inhibitors is that our founder, originally our founder, was studying kinase inhibitors in academic research.He was involved in the invention of the Kinase inhibitor "Fasil", which was sold in the world for the first time in the world, so he has commercialized its research with its history.
Also, the reason we specialize in this is that there are many kinase in the body, and in that sense it is a highly possible and market.In addition, there are quite a few opinions that "Kinase inhibitors can cure the whole body disease", but the eyes are relatively attractive organs in recent years and are important to improve the quality of life.Therefore, the most important purpose is to improve the quality of life with our technology and cure illness.
In addition, the eyes are very independent compared to systemic diseases, and because they are completed only with the eyes, it is possible to perform clinical trials for people as we do, so we can deliver medicine quickly to patients.I think it is possible.In addition, we specialize in ophthalmology with kinase inhibitors because there are relatively few competing companies and easy to do in business.
Hidaka: Next, I will introduce the business.It takes about 20 years to develop drugs, but the first part of the pharmaceutical research and development described in the slide, the beaker, the so -called world.The part that is being researched in various ways is called basic research, and it takes 5 to 8 years here, so it takes the first time.
After that, we will test with animals.This is said to be a non -clinical trial, but you will check the effects with animals, check the safety, and then move to a human exam.Clinical trials are also divided into the first and second semesters, and the phase 1, phase 2A in the previous term, phase 2b, and phase 3 in the second semester will be applied to the authorities and obtained approval.
We are making drug seeds by specializing in basic research and kinase inhibitors.Next, we will develop that kind of development, evaluate the effectiveness and safety of humans, and then develop business.In business development, we will develop what we have created in basic research, further improve the value of its development, and enhance our pipeline portfolio.We conduct efforts to increase corporate value through three businesses: basic research, clinical development, and business development.
Hidaka: About the company's work.After the establishment in 1999, it was listed on the Growth Market of TSE JASDAQ in 2009.In 2014, the drug invented by the company was first sold in Japan for the first time in Japan, and changed the strategy slightly after that year.Specifically, we have been conducting various initiatives since 2015 in order to promote the development of our own, the development of our own, clinical development, and the introduction of development costs conducted by other companies, based on the business form of basic research.rice field.
In addition, until 2014, we were doing business alone, but collaboration with other companies is also important, so we have been actively conducting joint research with other companies since 2018.
Shintaro Sakamoto (hereinafter referred to as Sakamoto): It was said that there are few bioventures specializing in ophthalmology.Considering the reason why your company is paying attention to the ophthalmology area, it is certainly good to fight in a place with few rivals, but since other companies have no technical infrastructure like your company, we do business in the ophthalmic area.Isn't it? Please tell us about this business environment.
Hidaka: For example, about 30 years ago, I think that the ophthalmic area was not very high in the medical industry compared to systemic diseases.In fact, in the development of drugs, there were not many companies that developed drugs approved for systemic diseases for eye diseases, that is, expand their adaptations, and developed in the ophthalmic area.
In that sense, it is probably possible to develop drugs using the technology that each company has in ophthalmology, but now ophthalmology has a unique know -how.Because there is a barrier behind the eyes and is independent with only the eyes, when trying to develop that eye, there is not much know -how that can be done immediately, and I think that it is a barrier to the entry.However, in recent years, ophthalmology drugs have been exploded, and I think that the number of feet has gradually increased.
Sakamoto: Thank you.I would like you to talk about the products created by your company around that.
Hidaka: About our outline and strengths.We have the city's invention and have a city item.It is said that there are about 2,000 bio ventures in Japan, with less than 60 bio ventures listed.Among them, there are several companies that have a city product, but there are few companies that have created and fulfilled the city.
In that sense, we are a very rare company, and we believe that basic technology has been proven.It's very big to take medicine in this industry, which is our strength.
Hidaka: About development pipeline.The slide lists a list of our development pipeline.It is divided into the upper and lower rows, the upper row is created by the company, the lower part is the introduction, and the introductions have been launched as business since 2014.
There is one arrow in the diagram, but since Kowa, a licensed outing for "K-232", applied for a manufacturing and sales approval on November 25, this is a little topic.Is put in.
Hidaka: The rest will be explained individually now.First of all, in -house products are mainly glaucoma in ophthalmology.Ripuszil hydrochloride hydrochlion is the general name of the active ingredient, but I would like to explain this.
It is said that the market in the whole world of glaucoma is about 600 billion yen, of which about 100 billion yen is Japan, and the number of patients will continue to increase in the future.In addition, although the number of eye drops is increasing, the number of medical devices called devices other than eye drops is gradually increasing, and it is a market where future growth is expected.
Glaucoma has a water called aqueous water flowing in the eyes, which causes the flowing or excessive production for some reason to increase the pressure in the eyes pressure, that is, the pressure in the eyes.is.
As a result, a disability occurs in the optic nerve, eventually narrowing the field of view, and finally blindness.Glaucoma is the number one cause of visual impairment in Japan, and is a serious disease that has been in the first place.Standard treatment is an eye drop, which is said to be important to lower the intraocular pressure, and can be delayed by administering eye drops and lowering intraocular pressure.
Among the eye drops, there is the first first selection drug used, which is said to be prostaglandin and PG -related drugs, which account for about 40 % to half the market.It has a very strong intraocular pressure drop.However, there are issues, and as described on the right side of the slide, it is difficult to reduce glaucoma with just one drug, even if it is the strongest drug.
Moreover, some patients are called non -less ponders who do not work on this PG -related drug, and the first selection is very important, but the disease is not easy to treat, so multiple drugs are used.is.
The Japanese market is a circular graph at the bottom right of the slide, but about 50 % of the place where "PG" is written and the "PG/β blocker" is written is the first selection drug, remaining.Half of the second and half after the second selection.
Sakamoto: I would like to ask a little, but in the treatment of glaucoma, at first, PG is probably used, and people who do not work in PG change from the next, and then change from the next.Please use this ", is it OK to use it in order for patients in a common manner?
Hidaka: You're right.
Sakamoto: If that doesn't work, it is common to use multiple treatments together.
Hidaka: Perhaps the first use is to use PG to control intraocular pressure, so it may not be too much to lower it too much.As you choose the medicine while looking at the site, you will choose the one that is for the patient, so it is quite difficult to have only one agent.
Sakamoto: It means that control around that is also difficult.I understand very well.It is a necessary medicine.
Hidaka: Among our development pipeline on glaucoma, the active ingredient names are repasgeil hydrochloride hydrate, and three are listed on the slides.First, "Granatech"."Granatech ophthalmic solution 0.4%is for glaucoma and hypertension, and has already been sold in Japan, approved in Asian countries listed on slides, and is applying in Vietnam.
This "Granatech" is a glaucoma -eye -catching drug that inhibits the world's first ROCK inhibitor, that is, RHO Kinase in Kinase, and its sales are steadily changing.Although the number of sales has not been announced, the peak sales are 7.6 billion yen in Japan among the materials applied to the authorities by Kowa, the license out, and we have received some of them as royalties.increase.
In addition to the glaucoma of "Granatech", there is "K-321" currently under development because it is expanding its adaptation.This subject is a disease called hook corneal endothelial degradation, and is still clinical in the United States, but ultimately a very serious disease that requires corneal transplantation.Since there is no treatment, we are currently conducting clinical trials in the United States, and if we get good results in the clinical trial, we believe that it is expected to be in the future.
Sakamoto: 6 million people are written, but it's a considerable number of people.
Hidaka: That's right.The maximum of about 4 million patients in the United States with glaucoma.
Sakamoto: That's more.
Hidaka: Yes.I think it will be a very large market if you can put in new drugs there.
Sakamoto: Is this the same in the world, or is it a disease that is common in white people?
Hidaka: It is a disease that is common in white people.
Sakamoto: Then, it's more European.
Hidaka: Kowa is clinical in the United States, as it is said that in Japan, which is not up to dozens of people, but it will fit in thousands of people.
Sakamoto: I understand well.In the future, your company has developed "Granatech", and there will be quite a lot of developments.This was 2014.
Hidaka: As you said, I went to Japan in Japan in 2014.As an enlarged adaptation, it is a hook corneal endothelial degradation and a combination agent.There is also a part that this "Granatech" alone has not been able to demonstrate the potential as an eye drop, so there are actually multiple drugs on site, so I applied for such things last week.did.
Sakamoto: To be blended means that the two types of eye drops have been OK for one type.
Hidaka: That's right.Usually, leave one type for 5 to 10 minutes after one type.
Sakamoto: You have to leave it.
Hidaka: I can now put it together.
Sakamoto: QOL rises considerably.
Hidaka: There are some.However, as for the improvement of QOL, there was also a circular graph in the market mentioned above, but if you look at the data, it is not surprisingly replaced, but I think that it often grows together.I think that the number of patients who use it will increase by using it for various purposes.
Sakamoto: That's interesting.
Hidaka: Then, about "H-1337".This target is also glaucoma, but it is developed with glaucoma and hypertopic disease because it is a POST-repaser hydrochloride hydrate.The phase 2A will be completed and phase 2b will be started in 2022 in the United States.What is different from Repus Jill in POST-Repus Jill is that we are developing in-house.
The Ripus Jill itself has been licensed to Kowa before entering clinical development, but this is developed by our company.As I will tell you later, we are doing business in the form of taking risks and getting profitable because it is more profitable to develop ourselves.
"H-1337" is an RHO kinase inhibitor and is an inhibitor called multi-kinase that inhibits multiple kinase.The disease I'm aiming for is glaucoma, and I mentioned earlier, but there are issues for standard treatment, where patients who do not work for PG.In the current situation where non -less ponder patients are using multiple treatments, we are aiming to make top lines used in second place.
Sakamoto: Then, the amount will increase overwhelmingly.
Hidaka: That's right.The market is half and half, so we believe that if you take the top there, there will be a market that will be on the first choice.
Sakamoto: It's very difficult to say more than PG, but PG is already generic, so it's no longer possible.
Hidaka: That's right.Cost performance is tough.
Sakamoto: I understand very well.
Hidaka: It is "DW-1001" because it is an introduction.It is difficult to explain this because it is licensed out of Rohto Pharmaceutical, and the details of the Rohto Pharmaceutical policy are not disclosed.
As for the information we are currently providing, phase 1 will start next year and phase 2 will be launched in 2023.It is a pipeline that uses a method called dragging positioning for ophthalmology disease.
We will still have more time to disclose, but this is a drug that has already been sold for non -eye diseases, and we are considering adding new adaptation with the eyes.As a result, the development risk is reduced.
Sakamoto: If the ophthalmology is adapted, the drug price may change, but will it be certified as a new drug?
Hidaka: That's right.It is approved as a completely new drug.
Hidaka: "DW-1002".Although it is a drug, it is a surgical assistant.The same is true for retinal separation, but it is called a vitreous surgery, and there is an operation to remove a pathological membrane attached to the vitreous body.The problem is that the border between membrane and vitreous body is very difficult to understand when resection.For example, it feels like a tissue paper on the gum and peeling it off, making it difficult to understand the border.
However, using the "DW-1002" will dye the membrane, making it easier to understand the site to be removed.Therefore, using such drugs to perform surgery more efficiently can make resection better.
Therefore, the use will increase according to the number of surgery.It is currently sold in Europe, the United States and Canada.As described in the slide, the estimated number of surgery is 100,000 in Europe and 200,000 in the United States.
It is not used by all patients, but we have a considerable number.Currently, phase 3 is over in Japan, and the watamoto pharmaceutical, which is the license out of the license out, is developing to apply for 2022.
In Japan, it may be used during cataract surgery.The number of cataracts is about 1.2 million, and it will be difficult if it is used in all of them, but it seems to be close to the lens and vitreous films mainly for patients with severe cataracts.It is used when there is something and it is necessary to remove it.
Such patients have less than 100,000 patients because they are less than 10 % of the total.In Japan, this disease is also developed.
Sakamoto: After inserting eye drops, it is not something you put in a knife and surgery, but to use it when there is a little more lump.
Hidaka: That's right.
Hidaka: "DW-5LBT".Medrex, a joint development start, is a company listed on the Tokyo Stock Exchange.This is a tape of a pain mitigation agent called lidocaine, but there is already "Lidoderm" as a preceding product.
Medrex was developing, with better adhesive strength, and less irritating skin irritation than "Lidoderm".On the other hand, we have contracted to develop jointly two years ago.
Ridocaine itself has a peak of about 100 billion yen, and the current estimated market is about 27 billion yen.The company plans to launch something very easy to use in this market and sell it.Currently, we are applying in the United States, and approval and sale will be after next year.
Hidaka: About the research project.For research projects, it will become our core project five years ahead and 10 years from now on how to do it in the last two to three years.
Currently, there are those who are studying at Mie University and those who collaborate in collaboration.Three projects are described in green on the slide, but this will be explained individually from the next page.As a whole, we make kinase inhibitors, and we are studying some other than ophthalmology and ophthalmology.
Hidaka: The first is a new device creation project.As I mentioned earlier in glaucoma, the glaucoma market itself has entered the market as well as eye drops.
Among them, the company called GLAUKOS is a company listed on the New York Stock Exchange, but has a very good device, and adds its excellent device technology and our kinase inhibitor to the market.We have been conducting joint research since 2018.
As a result, what happens is that, for example, an eye drops must be eye -eye once or twice a day, but surgical surgery..8 mm x 0.If you put a 5 mm device under your eyes, the drug will gradually be released from this device for about one and a half to two years to get the effect of the drug.
Sakamoto: Do you put it on the lower side of your eyes instead of stinging your eyes?
Hidaka: That's right.I think it's the lower side of my eyes.
Sakamoto: I was surprised.I thought, "It's absolutely painful to stab."
Iimura: It looks like science fiction, but how do you get out of such a small place?
Sakamoto: Is there a dark drug?
Hidaka: I will gradually take out the medicine gradually from that small device.
Iimura: That's strange.It looks like a manga story.
Hidaka: That's right.It requires a very good technology to gradually get out.We do not have that technology, and the partner who conducts joint research is.
Sakamoto: Does the provision of drugs adjust the depth?
Hidaka: In the case of eye drops, I put it every day, but the profile is slightly different to put the drug in that small device.You have to put a stronger drug.However, if you put more strong things, the side effects will be stronger.
Sakamoto: It's bad if you put it out too much.
Hidaka: That's right.それでは問題があるため、このデバイスに合ったもので、かつ効果をしっかりと得られるものを作らなければいけません。そのようなものをオーダーいただき、そのオーダーに合ったものを作って、それを一緒に開発していきます。
Iimura: The formulation is also extremely difficult.
Hidaka: That's right.
Sakamoto: This cannot be done unless you are making it.
Iimura: That's amazing.
Hidaka: The second is a slightly longer name, but it is a target protein decomposition induction drug development project.We make kinase inhibitors, but there are some good and bad kinase inhibitors.I wanted to be effective for better kinase, so I decided to decompose kinase.
Since the inhibition temporarily stops function, the decomposition breaks down the kinase itself, so theoretically, it has a stronger medicinal effect than the kinase inhibitor, and has a low dose and high safety.I think it will be expected.
As a background, a variety of technologies have been developed in the pharmaceutical industry in recent years, so if you do not import such things, you may not be able to fight in 5 or 10 years.We are making new efforts more and more.
We have been able to confirm the decomposition guidance effects of Kinase, and I would like to polish them and develop them as drugs.We do in collaboration with startup companies because we do not have the technology of disassembly induction.
Hidaka: The third is the AI drug discovery project.Our drug discovery is a craftsmanship, and we use human hands.In recent years, drug discovery using AI has been booming, and it is an attempt to create something unprecedented by collaborating with the technology using AI.
This is an initiative to create unprecedented kinase inhibitors for inflammatory and central systems other than ophthalmic diseases.It may be necessary for a very long period, but in the future, how to coexist with AI is probably a major theme of humanity, so we will use the roles and use the good points as they are.I'm going in I want.This is also a joint research with a company called SyntheticGestalt, because we have no AI technology.
These are the pipelines and projects.
Hidaka: From here on about the growth strategy.As our growth strategy, we aim to expand the development pipeline and expand our business area.
For this reason, in terms of expanding the development pipeline, we are considering securing future revenue sources and dispersing risks.In terms of expanding the business area, we hope to improve the amount of revenue and income and expand future sales.
How to proceed, it is described in the slide as "further improvement of quantity and quality" in the sense of expanding the development pipeline.For the time being, we are focusing on the amount.We are proceeding in the form of "Let's make new drugs", "Let's introduce them from the outside", and "expand adaptation and expand overseas."
In terms of expanding the business area, it is simple, but we do clinical development in -house.Because it costs money, you have to procure money before developing.Instead, we will aim for great profits in the future.
Hidaka: This is the data that is also disclosed.As a management indicator that emphasizes management, we aim to increase the number of development pipelines.I would like to add three pipelines in 2014 to 5 in 2019, 7 in 2020, and 10 in 2024.
The projects described earlier are listed in blue and orange in slides.There are six, but if you put three of these in clinical development, you can achieve 10, so I would like to put three in clinical development by 2024.
Sakamoto: I wanted to put 10 in clinical development for the time being, but please let me know if you have a plan, such as having enough personnel or having to increase the number of staff a little more.
Hidaka: If you proceed with 10 at the same time, you will need about three times the current person.However, since 2015, we have been conducting joint research and dividing the role with the company of joint research.In addition, all pipelines other than the "H-1337", which are currently developing in-house, have a partner.
It may not be much attention, but in that sense, it is a very limited part in that sense.So, if you do it in the current form, I don't think there is any problem with the personnel.
Sakamoto: I see.There is not much cost increase.
Hidaka: That's right.ただし、収益を目指してもっと多くのプロジェクトをどんどん行わなければいけないとなると話は別です。
Sakamoto: You need a lot of personnel and finance.
Hidaka: That's right.
Hidaka: Management indicators for expanding business areas are the progress of the development pipeline.The development plan for 2024 has been announced, but the plan is disclosed by the light blue part of the slide.I would like to proceed with this plan.
I want to make everything close to the city, but there are steps, so I would like to steadily proceed one by one based on the steps.
Sakamoto: Did the screening speed be slightly slower due to the new colon virus?
Hidaka: We don't talk directly to the authorities, and our partners talk, but in terms of being present there, it's not particularly slow.
Hidaka: Medium -term management plan.This is also announced, and it has been issued from sales to profits.R & D expenses may go up and down considerably, but we are planning the plan as described in the slide.
Hidaka: Slide has also issued a development plan.Looking at the sales, we are making upward revisions for 400 million yen this year, but after next year, we have been expecting sales in the range.
The company is planning to increase sales, but the reason is that the top city items surrounded by the light blue frame at the bottom of the slide will increase."DW-5LBT", "DW-1002 (Japan)" and "Granatech" are planned for these additions.We are planning an increase in sales because the number of Ushita will increase and the loyalty will increase.
Hidaka: About R & D expenses.The development pipeline plan is mainly a research project, but by promoting this project, you will need a lot of R & D expenses.
The biggest part is the "H-1337" phase 2B.The cost of developing in the United States is very large, which will cost you a lot.
Sakamoto: You already have the phase you are in the city, and I think that there is also a technology base.It is naturally deficit now, and it is expected to be in the red in the future, but if you have your skills, I think there is a product called "If this is the case, it will return everything unexpectedly and reverses one shot, and the shareholder is Banzai."I think "H-1337" is possible, but do you not take such a strategy other than that?
Hidaka: Probably all bio ventures in the world want to develop a blockbuster that sells more than 100 billion yen per drug.We think that too.So everyone is the same for such a thing.However, the aim is different for each company.I think that's a strategy.
We naturally want to hit one shot, but since they are listed, we explained earlier what kind of strategy to work on.However, pharmaceuticals have a low probability of success, so they will not succeed unless they.
We want to take a strategy of hitting one while hitting the numbers.In order to expand the pipeline, we have set the number of pipelines as a management indicator.It means aiming for quality after increasing the number.
I think that aiming for quality from the beginning is one strategy, and it is natural that it will succeed.That is the difference in how to choose.The biggest reason for choosing a number is that we have basic technology and can make new ones one after another.
We will hit the numbers and aim to create and hit the good quality.I guess it's a reversal of it, but I think the bio -venture managers probably think so.
We want to make things with high medical needs, so we want to aim for such things and make money.
Sakamoto: "I think the characteristic of the new drug candidate compounds is much higher than the average. The reasons are three compound library, drug design, and drag western method.However, please tell me why it led to differentiation. I think there is a reason why the introduction has a high barrier to entry. "
Hidaka: Our basic technology is characteristic of library.How difficult it is about the library, but the library we have and the original library is gold vein.Because we modify the gold vein and make it in various forms, there is a know -how in the way of the modifi.
We know how to dig gold vein, and there is a technology that can make new things one after another.The skeleton we are making is a 3D skeleton, but it captures the characteristics of this skeleton and is widely familiar with its skeleton.Sometimes you have a patent, but such a library is our best strength because there are situations where other companies try to enter there.
Iimura: I don't know if it's a liquid, but it was shocking to make a medicine to put in a small device.
Sakamoto: Is that a liquid?
Hidaka: That's right.
Sakamoto: I think there is a part that I talked about, but "Is there a possibility that you may advance to an area other than the ophthalmic area when expanding the business?Do you have any questions? "
Hidaka: We are in the ophthalmic area strategically.So, when you say, "It's an ophthalmology company," you really feel uncomfortable.In that sense, ophthalmic diseases have severe illnesses, so we want to make treatments for ophthalmic diseases with our technology.
On the other hand, from the viewpoint of kinase inhibitors, kinase inhibitors are often used for diseases that are not ophthalmology, and there are fewer companies that use kinase inhibitors for ophthalmology.So I want to work on diseases other than ophthalmology.
As one of the cuts, I am thinking of a project using the AI mentioned earlier.This is for inflammatory diseases and central diseases, but I think that it is difficult to work on a central system with a Japanese venture, but it also expands in such a place.I want to go.
I think it can be useful in terms of "Is the technology in the ophthalmic area useful for development?"Cells such as retina in the back of the eyes are nerve cells.Considering the cells in the brain, it is possible to use such know -how because there is a specificity that other organs do not have.However, other things do not use a lot, and I think that we will proceed with different diseases.